In order to measure the strength of a color, we use a machine called an absorbance spectrophotometer. The machine shines light through a cell containing a colorant solution and then measures the amount of light that is able to pass through the sample as compared to the original amount of light from the source.
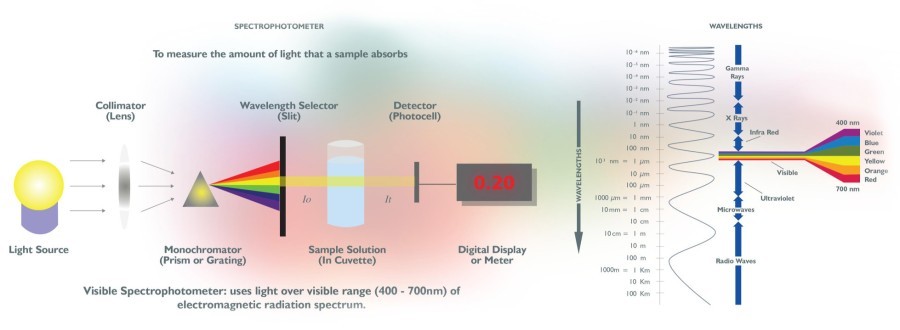
What does this show? A colorant with a very high concentration will only allow a very small amount of light to pass through due to the density of the colorant, while a lower concentration colorant will absorb much less of the light source, allowing more light to pass through to the photocell detector.
Additionally, the spectrophotometer uses light across the entire range of the visible spectrum. In this way the photocell detector not only detects how much light has passed through, but it also detects the wavelengths of the light that passes through. Before it reaches the sample solution, the light passes through a monochromater that divides and diffracts the light much in the same way a prism does, separating the entire visible spectrum of light into each individual color wavelength, giving even more in-depth analysis of the strength and composition of the color concentrate.
